Solution & Reactive Adhesives
 There is no universal adhesive that will fulfill every application, and it is usually necessary to compromise when selecting a practical adhesive system however, understanding the basic differences between the solution and reaction adhesives should equip the preparator with some of the fundamental information necessary to make the most appropriate and successful choice.
There is no universal adhesive that will fulfill every application, and it is usually necessary to compromise when selecting a practical adhesive system however, understanding the basic differences between the solution and reaction adhesives should equip the preparator with some of the fundamental information necessary to make the most appropriate and successful choice.
There is a fundamental difference between the structure of the polymers formed by solution and reaction adhesives.
Solution adhesives
Solution adhesives are linear or slightly branched polymers. This means that the monomer units of which they are formed are strung together in straight chains [insert adhesives Fig. 3], which sometimes have small chains or side branches. They are applied as pre-made linear molecules that do not change their basic chemistry or structure as they set or dry.
Solution adhesives such as Paraloid B-72, Butvar B-76, B-98, and McGean B-15 are often purchased as solid polymeric materials (in the form of powders or beads) that can be mixed with organic solvents such as acetone or ethanol to form liquids for application. Both the molecules of the liquid solvent and those of the solid polymers are held together with weak secondary bonds. If the attractive forces within the polymeric material are weaker than those between the solvent and the polymer, the polymer molecules will be pulled apart and go into solution. The linear polymer molecules are still intact but are separated and floating in the solvent like strands of pasta in water.
As the solvent evaporates the polymer strands come into closer contact, re-establishing secondary bonds with each other and also becoming physically entangled, forming a solid mass [insert Fig. 5]. If solvents are reapplied at a later date the chains can still untangle and separate again forming a liquid. Some solution adhesives can be re-dissolved in this way repeatedly and indefinitely, because the polymeric material remains chemically unchanged before and after “setting”.
Reaction adhesives
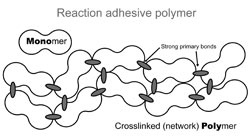
Reaction adhesives, on the other hand, are applied as liquid monomers that chemically react to form solid polymers (polymerize) in place after application. The resulting structure is a network, connected or cross-linked with primary bonds [insert Fig. 4]. In other words, the solution adhesives remain a collection of individual molecules, while reaction adhesives basically form one large polymer molecule.
Reaction adhesives such as epoxies (Devcon, Epo-Tek, etc) and cyanoacrylates (Aron Alpha, Paleo-bond, etc) are purchased as liquid monomers which chemically react after application to form networked polymers (Fig. 6). However, epoxies and cyanoacrylates form these structures in different ways:
oEpoxies are sold as two separate liquids: a resin and a hardener, which chemically react to form the network polymer when they are mixed together.
oCyanoacrylates are sold as a single liquid which is a monomer, usually combined with an acid which prevents formation of the polymer before application. When the monomer comes in contact with the trace moisture naturally present on the surfaces to which it is applied, the acid is neutralized and polymerization occurs.
Both types of reaction adhesives undergo chemical change in the solidification or setting process, and unlike solution adhesives, strong primary bonds are formed. Once set, the resulting material cannot easily be dissolved or broken down. Some organic solvents may swell or soften the structure making it easier to break it apart physically, but commonly used organic solvents, such as acetone and ethanol, will be unable to separate the strong primary bonds holding these networked polymers together.
Working properties of solution and reaction adhesives
Working and setting times
Working time refers to the amount of time that the preparatory can manipulate the adhesive or the join. Setting time refers to the amount of time necessary until the adhesives if fully dry or cured and has reached its full strength.
Solution adhesives
The working time and set time of solution adhesives are dependent on the volatility of the solvent used. The solution adhesives mentioned here can be dissolved in a range of different organic solvents with diverse rates of evaporation. This property can be exploited to vary the working and setting time of an adhesive to meet the requirements of a specific task. Conveniently, acetone and ethanol, the two solvents most commonly found in the prep lab and field, present a range of volatility from fast (acetone) to moderate (ethanol). Temperature and air flow can also affect working times of adhesives that set by evaporation; evaporation can be slowed by covering the specimen to reduce air-flow, and these adhesives will set more quickly if used out in the hot sun in the field. Working time of solution adhesives can also be affected by volume; application of tiny drops for micropreparation can be difficult because they harden too quickly due to a high surface-to-volume ratio which speeds evaporation. It may take a long time for a solvent based adhesive or white glue emulsion to fully dry.
Reaction adhesives
Epoxies – Epoxies naturally set slowly and the long working time of epoxy allows plenty of time to align and adjust fragments before setting. This can be useful for small, complex joins such as multiple broken cusps on small teeth. Epoxies can be used for consolidation of fine cracks; although viscous they can penetrate well because they have slow set times which allow them more time to flow. This is especially true of very slow setting epoxies (Epo-Tek 301-2, Hxtal NYL-1) which can take days to set. Epoxies with very fast set times, such as 5 minute formulas, have added accelerators and these additives can lead to an inferior product.
Cyanoacrylates – Most set relatively quickly and are thus sometimes preferred when clamping is not possible. Because polymerization is initiated by surface moisture, cyanoacrylates do not usually harden until they make contact with the substrate, thus they can be applied as tiny drops. They also set more slowly in dry conditions and more rapidly in humid conditions, which is why some preparators speed setting with their breath.
.
Viscosity
Viscosity is defined as the resistance of a liquid to flow. The more viscous the adhesive, the thicker it will be and the slower it will pour and spread.
Solution adhesives
The viscosities of solution adhesives can be modified easily by adjusting the concentration of the polymer in the given solvent. Paraloid B-72 can be mixed in concentrations as low as 1-5% to produce a dilute low viscosity resin for consolidation, or in concentrations as high as 35-50% to produce a thick adhesive for joins. A high viscosity mixture of Paraloid B-72 in acetone loaded into a tube, commonly used by conservators, is very useful and convenient for quick assembly of fragments, and can often be used in place of commercially packaged fast setting epoxies and cyanoacrylates, which while convenient, are not as resoluble and do not have as good aging properties as Paraloid B-72. One problem with solution adhesives is that their viscosity is directly linked to their concentration or polymer content. The only way to produce a low viscosity adhesive is to have a dilute or low concentration solution; one may get the adhesive solution into place but once the solvent evaporates relatively little actual adhesive polymer remains.
Reaction adhesives
Because reaction adhesives do not contain solvent, it is possible to have low viscosity epoxies and cyanoacrylates with 100% monomer content, all of which reacts to form the polymer. This can be advantageous for very small joins and hairline cracks, where the need is to get a significant amount of adhesive into a very small space.
Penetration and migration
Penetration refers to how deeply the adhesive travels into the fossil substrate and migration refers to movement, generally back towards the surface, generally during setting. In some cases deep penetration of the adhesive may not be necessary to achieve adequate consolidation, as superficial consolidation with more stable solution adhesives is often very effective at binding together difficult material in the field and the lab.
Solution adhesives
The volatile solvent component of solution adhesives causes the polymer to migrate during solidification. The solvent is the carrier for the polymer: it carries it in, but also carries it back out as it evaporates and the polymer can often end up deposited on or close to the surface. The propensity of solution adhesives to migrate to the surface with the solvent can be problematic when one is trying to achieve deep consolidation. Migration of solution adhesives can be moderated by a variety of factors that have been discussed in the conservation literature, including solvent selection and control of drying conditions.
Reaction adhesives
Reaction adhesives have no volatile solvent component and thus do not have a tendency to migrate out after penetration. They are applied as liquids composed of monomers, which are much smaller, more compact molecules than the linear or branched chain polymers of the solution adhesives. Thus they are more able to travel into the open spaces in the substrate, potentially achieving deeper penetration. This is especially true of cyanoacrylates which are not only composed of small monomer molecules but can also have very low viscosities without being dilute. However, low viscosity cyanoacrylates are also known to have poor gap filling properties. Thus they may achieve deep penetration without successful consolidation in instances where there are large cracks or voids that need to be filled in order to stabilize the specimen effectively. Reaction adhesives migrate less and have the potential to penetrate better than solution adhesives, but their insolubility, hardness, and questionable aging characteristics may outweigh these advantages.
Final properties of solution and reaction adhesives
Resolubility
The ability of the adhesive to be reversed or dissolved after fully setting.
How do the two types of adhesives react to solvents?
Solution adhesives – The organic solvents commonly used by fossil preparators (e.g., acetone, ethanol) can easily redissolve solution adhesives as they are held together with weak secondary bonds.
Reaction adhesives – organic solvents are not effective on reaction adhesives which are held together by strongly linked primary bonds. There are some instances when reaction adhesives can be softened with solvents and removed successfully, such as when they are used on a very small scale. However, in almost all cases removing or reducing reaction adhesives will require more time, effort, and far greater risk to the fossil.
Why is resolubility advantageous?
Resolubility makes adhesives much easier to rework and remove. This is especially advantageous for consolidants applied in the field and during preparation in the lab, which are often eventually discarded with the excess matrix. Resolubility is also advantageous in the long-term. Fossils are very commonly repaired, disassembled, stripped of coatings, and re-prepared for molding, display or for research; the future uses and requirements of the fossil are not always foreseeable so, when possible, it is always preferable to use something reversible or reworkable.
When is resolubility not advantageous?
There are some rare cases where resolubility may be undesirable, such as smaller, more delicate joins that could dissolve by accident if the surface was exposed to a solvent during cleaning or application of a coating in preparation for molding. More commonly, there can be instances where reversibility is sacrificed because reaction adhesives offer properties not available with solution adhesives, such as the ability to penetrate into hairline cracks or great strength.
In cases where a strong but insoluble adhesive such as an epoxy is necessary (e.g.,for joining heavy elements) it is sometimes possible to use this in combination with a soluble adhesive. For example, barrier layers of Paraloid B-72 can be applied prior to application of an epoxy in order to allow for greater reversibility of the join in the future. It has been shown that this technique, if executed properly, can be used without negatively impacting the strength of the join. Soluble barrier layers can also be used to increase reversibility when epoxy putties are used to fill gaps.
Strength & flexibility
Strength, in this context, is the ability of the adhesive to hold a join without slumping or sagging over time. The ability to withstand pulling without breaking is referred to as tensile strength and the ability to withstand pushing forces is compressive strength. Flexibility refers to the ability of the adhesive to withstand some movement without breaking the join.
How do the two types of adhesives react to gravity and other outside forces?
Solution adhesives – Solution adhesives are somewhat softer and more flexible than reaction adhesives due to their weaker secondary bonding.
Reaction adhesives – Generally, the primary bonded, reaction adhesives are harder and more rigid with greater cohesive strength.
When is adhesive strength advantageous?
When the joined parts are so large or heavy that more resoluble solution adhesives might fail due to the stresses of gravity over time, the strength of epoxies can be an advantage. The hardness and rigidity of reaction adhesives also makes them useful when specimens must withstand extreme stresses during preparation, such as the impact of a chisel or powerful air scribe.
When is adhesive strength not advantageous?
Stronger is not always better. The relative “weakness” of solution adhesives can be advantageous in some cases. In addition to being resoluble, these adhesives require less force to remove mechanically without the aid of solvents. Thus solution adhesives often work better than reaction adhesives when preparation requires temporary consolidation of loose matrix or application of temporary coatings which will later be removed with needles or air scribes.
In addition, very hard and rigid adhesives like epoxies and cyanoacrylates generally lack elasticity or flexibility unless they are heavily modified with additives. The ability to give or stretch under strain can be an important quality in a successful adhesive, as it allows it to move and bounce back under certain forms of stress rather than breaking or transferring the stress to the object and potentially causing damage. Generally it is undesirable for an adhesive to be more rigid or harder than the substrate as this can lead to damage in the original material. If the adhesive used in a join has more cohesive strength than the fossil itself, applied stress may fracture the fossil rather than the adhesive, resulting in characteristic fresh breaks parallel to the original join. Similarly, consolidation of soft substrates with very hard adhesives can cause zones of weakness due to incomplete and uneven penetration. Flexibility is a particularly important consideration when selecting an adhesive for use on sub-fossil or other materials that may expand and contract in reaction to fluctuating environmental conditions, such as relative humidity.
Aging
When adhesives are used for long-term applications it is preferable that their aging properties be proven and well understood. Poor aging of an adhesive can lead to a variety of undesirable results including shrinkage, distortion, embrittlement, decreased solubility, and darkening or yellowing over time. Damage from poor aging of adhesives can be found in most fossil collections, often including join failures, and embrittled, lifting coatings that have damaged the surface of the bone.
How do the two types of adhesives age?
Solution adhesives – When solution adhesives are purchased as powders or beads they are generally single ingredient products, containing only the pure polymeric material. This makes it easier to assess and predict their aging characteristics, and many solution adhesives are known to have excellent aging properties.
- Paraloid B-72
- Polyvinyl acetates - are reportedly somewhat less stable than Paraloid B-72 but are still generally considered to have very good aging properties.
- Polyvinyl butyrals – The long-term aging of PVBs has been questioned in the past but recent studies indicate it is also a very stable material.
- Emulsions or “white glues” - These adhesives consist of minute particles of non-water soluble polymers such as polyvinyl acetates or acrylics which are suspended in an aqueous solution. They set by evaporation of water, but are water soluble only before they are fully set. Once set, the resulting polymer film is only soluble in non-aqueous solvents such as acetone, toluene, and xylene. These adhesives are complex formulations as their suspended state is achieved and maintained through the addition of various materials such as emulsifiers, stabilizers and dispersing agents, and the compositions often include many other additives including plasticizers, thickening agents, and biocides. The quality of these adhesives varies greatly. The formulations commonly sold for home use (e.g. Elmers Glue-All) can become hard, brittle, discolored, and insoluble over time. Other specialty formulations of emulsions or dispersions, particularly the acrylics, may fare better over time, including certain grades of adhesives with the trade names Acrysol, Primal, Rhoplex, Jade and others. However, even these “better quality” white glues are general acidic and only recommended for use when a water based adhesive is required, such as in the consolidation of wet specimens in the field.
- Cellulose nitrate - often found in household glues such as Duco Cement, has poor ageing properties becoming very brittle, shrinking, yellowing, and weakenking with age, often leading to bond failure.
- Other natural and synthetic resins - Other solution adhesives can become harder and less soluble with time by cross-linking (forming primary bonds), like reaction adhesives. This is true of some natural resins such as shellac and also some modern synthetic resins such as Paraloid B-67, which is sometimes used as a resistant coating for acid preparation.
Reaction adhesives – In contrast to the solution adhesives, reaction adhesives are often fairly complex formulations, with ingredients that can vary from one manufacturer to another. Formulas can change according availability and cost of ingredients, and they often include additives that can affect aging. Even in formulations with fewer additives, it is difficult to know exactly what you are using: the terms “epoxy” and “cyanoacrylate” both denote a large and varied category of resins. The materials within these categories share some basic chemistry, but may differ significantly in their properties, which can make it more difficult to make general statements about many aspects of their behavior including aging.
- Epoxies - The aging of epoxies has been studied in connection to the conservation of glass and many formulations have been found to yellow severely. Yellowing is generally considered a sign of degradation and may be indicative of other changes in the material over time. The epoxies that have been shown to yellow the least are those with few additives that have been formulated for use in art conservation or for optical applications, such as Hxtal NYL-1 and Epo-Tek 301-2. While these epoxies have very long set times that make them impractical for many preparation tasks, others with more reasonable setting times have also been shown to be relatively stable and have found use in the conservation of stone, including: Araldite AY103/HY991. All epoxies are subject to user error: imprecise measurement of the components and inadequate mixing can interfere with the chemical reaction, resulting in incomplete polymerization and poor aging. This is especially true of very fast-set epoxies such as 5-minute formulas that can harden before the components have a chance to fully react. These fast-set formulas may contain additives that make them quick, easy, and convenient for casual consumer use, but make them poor choices when the goal is producing the best possible bond with the most predictable behavior over time (Horie, 1987: 173). In addition, all epoxies have a very limited shelf-life, of about one year. Epoxies that are past their shelf life may appear to set after mixing but may not have polymerized properly and might eventually deteriorate, so it is always preferable to discard old epoxies and to use freshly received material.
- Cyanoacrylates - have found increasingly wide use in fossil preparation since the late 1970’s. However, the aging behavior of cyanoacrylates has not been fully investigated. This is partially attributable to the fact that they have not been used widely in the field of art conservation, which has initiated many of the previous assessments of adhesive stability applicable to fossil preparation. One published study of cyanoacrylates has shown that there are still many unanswered questions regarding their stability and that contact with some fossils may accelerate degradation of cyanoacrylates. The unique properties of cyanoacrylates, such as their superior ability to penetrate into hairline cracks and fast set time, may override questions about their aging in some instances, but care should be taken to avoid using them when more fully investigated adhesives could do the same job.
Resources
The bulk of the text on this and the other pages on this topic was excerpted and adapted from An Introduction To Solution And Reaction Adhesives For Fossil Preparation by Amy Davidson and Samantha Alderson published in Methods in Fossil Preparation Proceedings of the First Annual Fossil Preparation and Collections Symposium, edited by Matthew A. Brown, John F. Kane, and William G. Parker. Petrified Forest, 2009. To read the complete text and access the specific citations download the full article.
- To learn more about the other talks given at the symposium visit the National Park Service Petrified Forest Fossil Preparation and Collections Symposium page.
- Click here to buy a copy of the published proceedings.
- Download the document Adhesives and Adhesion by Jonathan Thornton, a professor in the conservation program at Buffalo State College. This document covers topics including: History, Terminology, Choice of Adhesive, and gives information on a wide range of specific adhesives used in conservation.
- Learn more in the distance learning course Chemistry for Conservators. This course, taught by Velson Horie a leading expert in the field, is available through International Academic Projects. Download Horie’s useful Adhesives glossary here.
- Download Adhesives and consolidants in geological and paleontological applications; part one: introduction, guide, health and safety, definitions and part two: wall chart. Society for the Preservation of Natural History Collections. 1997. vol 1 leaflet 2. By Ann Elder, Scott Madsen, Gregory Brown, Carrie Herbel, Chris Collins, Sarah Whelan, Cathy Wenz, Samantha Alderson and Lisa Kronthal.
- Read a case study by AMNH preparator Amy Davidson on The use of cyanoacrylates and Butvar B-76 (polyvinyl butyral) on a specimen of Shuvuuia deserti (IGM 100/977) from Ukhaa Tolgod, Gobi Desert, Mongoliaabout and download the associated handout.
- Read more about why 5-minute epoxy might not be the best choice for use in fossil preparation….

